
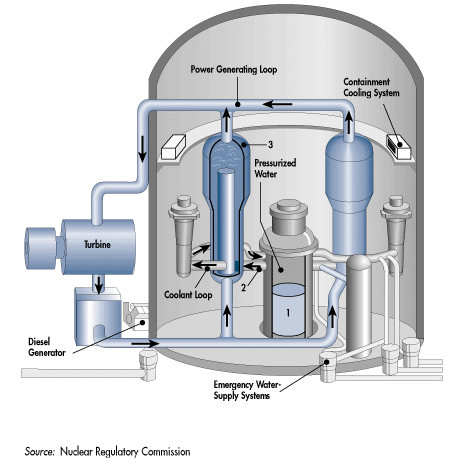
The term isotope applies to both stable and radioactive isotopes. The time it takes for one half of the atoms of a given isotope in a sample to decay is called the half-life, symbol t 1/2, of that isotope. An unstable isotope (also called a radioactive isotope or radioisotope) is energetically unstable and will decay (disintegrate) over time to another isotope of the same element or to a nuclide of a different element. A stable isotope is defined as an isotope for which no radioactive decay has been experimentally detected. 64Ni, with 28 protons and 36 neutrons, is stable, whereas 65Ni, with 37 neutrons, is unstable. For any particular element, only certain isotopes are stable. The term “isotope” is commonly used in discussions of atomic properties of an atom and “nuclide” is used for discussions of nuclear properties. Nuclides of a given element that have different numbers of neutrons, but the same number of protons, are called isotopic nuclides or isotopes. The terms Nickel-64 and 64Ni both refer to a nuclide of the element nickel with a mass number of 64. A nuclide is an atom with a specific number of protons and a specific number of neutrons that is, a specific atomic number and mass number. The total number of protons and neutrons ( Z+ N) in a specific atom is the mass number, symbol A, where A= Z+ N. The number of neutrons, symbol N, in an atom of a given element may vary. The number of protons in each atom is its atomic number, symbol Z, and determines the chemical element for example, for hydrogen atoms Z=1 and for gold atoms Z=79. Ītoms of all chemical elements are composed of positively charged particles called protons, an equal number of negatively charged particles called electrons, and electrically neutral particles called neutrons. IUPAC Periodic Table of the Elements and Isotopes. Practical applications of isotopic measurements and technologies are included for the following fields: forensic science, geochronology, Earth-system sciences, environmental science, and human health sciences, including medical diagnosis and treatment. An element-by-element review accompanies the IPTEI and includes a chart of all known stable and radioactive isotopes for each element. The background color scheme of cells categorizes the 118 elements into four groups: (1) white indicates the element has no standard atomic weight, (2) blue indicates the element has only one isotope that is used to determine its standard atomic weight, which is given as a single value with an uncertainty, (3) yellow indicates the element has two or more isotopes that are used to determine its standard atomic weight, which is given as a single value with an uncertainty, and (4) pink indicates the element has a well-documented variation in its atomic weight, and the standard atomic weight is expressed as an interval. Color-coded pie charts in each element cell display the stable isotopes and the relatively long-lived radioactive isotopes having characteristic terrestrial isotopic compositions that determine the standard atomic weight of each element. Each cell of the IPTEI provides the chemical name, symbol, atomic number, and standard atomic weight of an element. The IPTEI is intended to hang on the walls of chemistry laboratories and classrooms. The IPTEI is modeled on the familiar Periodic Table of the Chemical Elements.

The IUPAC (International Union of Pure and Applied Chemistry) Periodic Table of the Elements and Isotopes (IPTEI) was created to familiarize students, teachers, and non-professionals with the existence and importance of isotopes of the chemical elements.


 0 kommentar(er)
0 kommentar(er)
